Medica
FUJIFILM Holdings' Chairman Kenji Sukeno Awarded Japan's Order of the Rising Sun, Gold and Silver Star
FUJIFILM Diosynth Biotechnologies Takes a Major Step Forward in Global Expansion
Fujifilm Announces Robust Financial Results for the First Half of Fiscal Year 2024
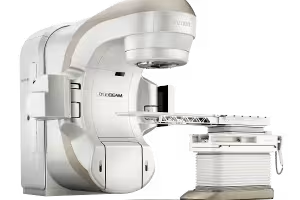
Varian Medical Systems: Pioneering the Fight Against Cancer with TrueBeam Radiotherapy System
Building Strong Patient-Provider Relationships for Lasting Contact Lens Satisfaction
SHERIDAN, WYOMING – November 9, 2024 – When it comes to contact lens wear, open communication between eye care providers and patients is essential for achieving lasting satisfaction and optimal eye health. Alcon, a global leader in eye care, recognizes the importance of this relationship and emphasizes the value of in-depth discussions to address patient needs and concerns.
Understanding Patient Priorities
bioMérieux's New VIDAS® Test Revolutionizes Vitamin B12 Deficiency Diagnosis
Alcon Showcases Cutting-Edge Innovations in Ophthalmology at AAO 2024
SHERIDAN, WYOMING – November 9, 2024 – Alcon, a global leader in eye care dedicated to helping people see brilliantly, presented its latest innovations and clinical data at the American Academy of Ophthalmology (AAO) 2024 annual meeting. The company showcased advancements in glaucoma therapy, retina surgery, and dry eye treatment, demonstrating its commitment to improving patient outcomes and streamlining surgical workflows.
Voyager™ DSLT: Revolutionizing Glaucoma Treatment